What Is The Formula For Magnesium Sulfide
A chemical formula shows the symbols of the elements in the compound and the ratio of the elements to one another. Although its formula (h 2 o) seems simple, water exhibits very complex chemical and physical properties. For example, its melting point, 0 °c (32 °f), and boiling point, 100 °c (212 °f), are much higher than would be expected by comparison with analogous compounds, such as hydrogen sulfide and ammonia. Empirical formulae are usually obtained based on the analysis of experimental data. These are limited to a single typographic line of symbols, …

Although its formula (h 2 o) seems simple, water exhibits very complex chemical and physical properties.
The empirical formula for glucose is ch 2 o. Remember that metals tend to lose their electrons, falling back to their inner octet, becoming smaller, forming positive "cations". Chemical formulas can be quite simple sometimes as h (hydrogen), or it can take a rather complicated form, such as ch 3 ch 2 oh (ethanol). Empirical formulae can be derived from the molecular formulae. All the atoms that became ions did so because they were losing or gaining enough electrons to become stable like a noble gas. It is an important precursor to other barium compounds including baco 3 and the pigment lithopone, zns/baso 4. As the name suggests, the structural formula of a chemical compound provides insight into the arrangement of the atoms in the molecule. A chemical formula shows the symbols of the elements in the compound and the ratio of the elements to one another. In its solid form, ice, water is less. Bas is the barium compound produced on the largest scale. Like other chalcogenides of the alkaline earth metals, bas is a short wavelength emitter for electronic displays. Using this program will help you to learn how to write ionic compound names and formulas for chemistry a. Magnesium magnesium ion fluorine fluoride ion sulfur sulfide ion nitrogen nitride ion 3.
All the atoms that became ions did so because they were losing or gaining enough electrons to become stable like a noble gas. Remember that metals tend to lose their electrons, falling back to their inner octet, becoming smaller, forming positive "cations". Empirical formulae can be derived from the molecular formulae. Empirical formulae are usually obtained based on the analysis of experimental data. A chemical formula shows the symbols of the elements in the compound and the ratio of the elements to one another.
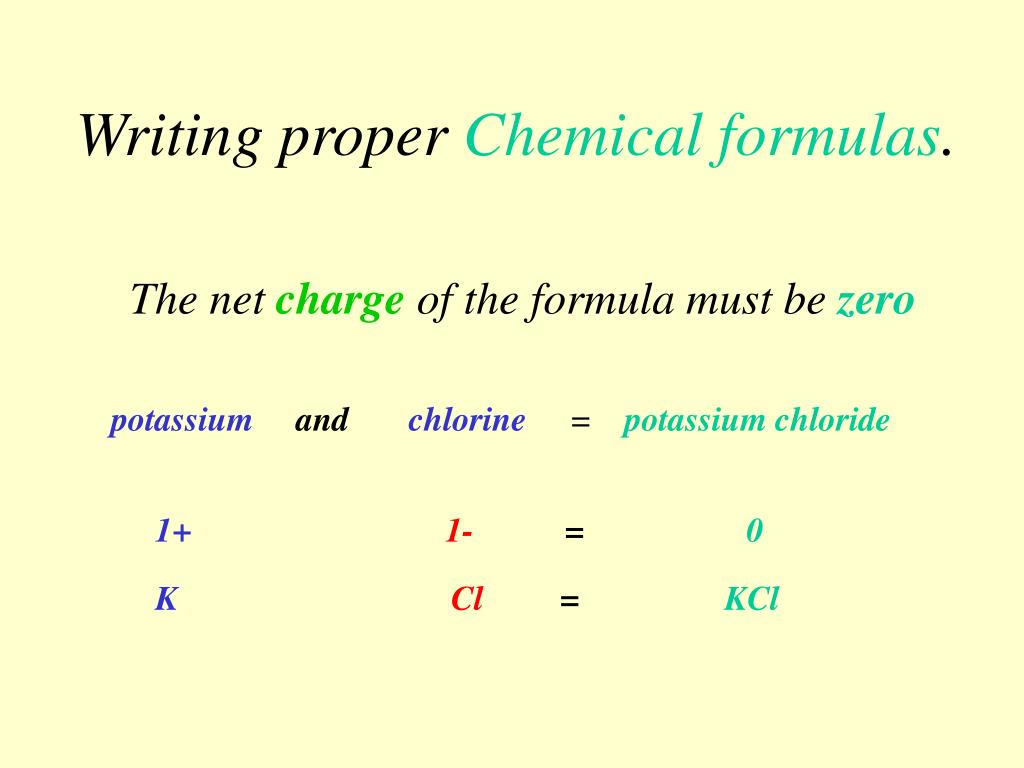
These are limited to a single typographic line of symbols, …
Look back at question #2. All the atoms that became ions did so because they were losing or gaining enough electrons to become stable like a noble gas. Like other chalcogenides of the alkaline earth metals, bas is a short wavelength emitter for electronic displays. Formula 21) sodium phosphide na 3 p 22) magnesium nitrate mg(no 3) 2 23) lead (ii) sulfite pbso 3 24) calcium phosphate ca 3 (po 4) 3 25) ammonium sulfate (nh 4) 2 so 4 26) silver cyanide agcn 27) aluminum sulfide al 2 s 3 28) beryllium chloride becl 2 29) copper (i) arsenide cu 3 as 30) iron (iii) oxide fe 2 o 3 Although its formula (h 2 o) seems simple, water exhibits very complex chemical and physical properties. The empirical formula for glucose is ch 2 o. Remember that metals tend to lose their electrons, falling back to their inner octet, becoming smaller, forming positive "cations". A chemical formula shows the symbols of the elements in the compound and the ratio of the elements to one another. A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and plus (+) and minus (−) signs. Bas is the barium compound produced on the largest scale. As the name suggests, the structural formula of a chemical compound provides insight into the arrangement of the atoms in the molecule. Chemical formulas can be quite simple sometimes as h (hydrogen), or it can take a rather complicated form, such as ch 3 ch 2 oh (ethanol). In its solid form, ice, water is less.
Remember that metals tend to lose their electrons, falling back to their inner octet, becoming smaller, forming positive "cations". Empirical formulae can be derived from the molecular formulae. Although its formula (h 2 o) seems simple, water exhibits very complex chemical and physical properties. Empirical formulae are usually obtained based on the analysis of experimental data. These are limited to a single typographic line of symbols, …
A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and plus (+) and minus (−) signs.
Look back at question #2. All the atoms that became ions did so because they were losing or gaining enough electrons to become stable like a noble gas. In its solid form, ice, water is less. Empirical formulae are usually obtained based on the analysis of experimental data. For example, its melting point, 0 °c (32 °f), and boiling point, 100 °c (212 °f), are much higher than would be expected by comparison with analogous compounds, such as hydrogen sulfide and ammonia. Empirical formulae can be derived from the molecular formulae. A chemical formula shows the symbols of the elements in the compound and the ratio of the elements to one another. Formula 21) sodium phosphide na 3 p 22) magnesium nitrate mg(no 3) 2 23) lead (ii) sulfite pbso 3 24) calcium phosphate ca 3 (po 4) 3 25) ammonium sulfate (nh 4) 2 so 4 26) silver cyanide agcn 27) aluminum sulfide al 2 s 3 28) beryllium chloride becl 2 29) copper (i) arsenide cu 3 as 30) iron (iii) oxide fe 2 o 3 Nonmetals tend to gain electrons, filling up their current energy levels, becoming larger, forming negative "anions". Although its formula (h 2 o) seems simple, water exhibits very complex chemical and physical properties. Remember that metals tend to lose their electrons, falling back to their inner octet, becoming smaller, forming positive "cations". Magnesium magnesium ion fluorine fluoride ion sulfur sulfide ion nitrogen nitride ion 3. As the name suggests, the structural formula of a chemical compound provides insight into the arrangement of the atoms in the molecule.
What Is The Formula For Magnesium Sulfide. Although its formula (h 2 o) seems simple, water exhibits very complex chemical and physical properties. Empirical formulae can be derived from the molecular formulae. In its solid form, ice, water is less. Nonmetals tend to gain electrons, filling up their current energy levels, becoming larger, forming negative "anions". Like other chalcogenides of the alkaline earth metals, bas is a short wavelength emitter for electronic displays.
Post a Comment for "What Is The Formula For Magnesium Sulfide"